One of the major obstacles using conventional cancer therapies such as radiotherapy or chemotherapy is the lack of specificity for the cancer cells. Ionising radiation and cytotoxic drugs effect both normal and tumour tissue, and damage to normal tissue limits the doses that can be used.
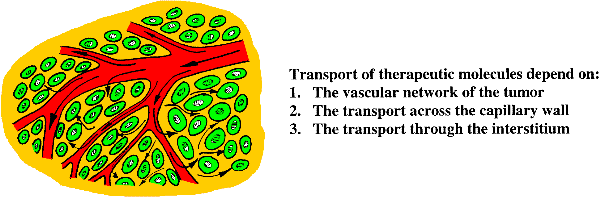
Various strategies to develop tumour selective therapies have thus been proposed. Monoclonal antibodies binding to tumour specific antigens on the cell surface can be used as carrier for therapeutic agents such as radionuclides, toxins or other drugs. Liposomes carrying drugs are another tumour selective therapy. Liposomes improve the pharmacokinetics, reduce the toxicity to normal tissue and increase the selectivity to tumour tissue compared to free drug, due to the leaky tumour capillaries. Gene therapy using DNA vectors carrying potentially therapeutic genes might become another cancer specific therapy. The success of the humane genome project has made it possible to diagnose errors in genes. The next challenge is to develop therapies based on this knowledge. The first hurdle in gene therapy is the gene delivery tools and barriers. All these tumour-specific therapies use rather large molecules with diameters in the order of 10 to 10.000 nm, whereas conventional chemotherapeutic agents are small molecules below 1 nm in diameter. The large molecules face severe problems reaching the tumour cells and only a minor fraction of the injected therapeutic macromolecules have been shown to accumulate in the tumour tissue (Davies et al 2004, Eikenes et al 2004). When injected intravenously or given orally the therapeutic agents have a complex journey. The success in reaching and killing the cancer cells depends on the vascular network of the tumour, the permeability of the capillary wall, and the ability to penetrate through the interstitium in concentrations high enough to kill the tumour cells (Jain 2001).
The vascular network in the tumour is often insufficient for an adequate distribution of therapeutic molecules, as the proliferation of the tumour cells exceed the proliferation of the vascular and stromal elements. Elevated geometric and viscous resistance in tumour vessels due to the irregular morphology of the vessels, induces a temporal and spatial heterogeneity in tumour blood flow (Jain 1988). These factors contribute to a reduced delivery of the therapeutic agents. On the other hand the permeability of tumour capillaries is shown to be higher than in normal capillaries, and these leaky capillaries thus favour the transport of the therapeutic agents across the blood vessel (Davies et al 2004, Yuan 1998).
The transport across the blood vessel wall as well as through the interstitium are governed by convection (driven by the hydrostatic pressure gradient) and diffusion (driven by the concentration gradient). A major obstacle to accumulation of therapeutic macromolecules in tumour tissue is the high interstitial fluid pressure. This pressure is shown to be higher in tumour tissue than in normal tissue, and a step pressure gradient is reported at the periphery of the tumour (Boucher et al 1990, 1992, Brekken et al 2000, Eikenes et al 2004). The transport across the capillaries might thus be more pronounced at the periphery than in the interior of the tumour (Davies et al 2004, Eikenes et al 2004, 2005). The high interstitial fluid pressure also impedes the penetration through the interstitium. Within the tumour the interstitial fluid pressure might be about equal to the pressure in the blood vessel, hence convection virtually ceases. The main mechanism to reach the tumour cells is thus by diffusion. Diffusion depends strongly on the size of the molecule, and for large molecules the process is extremely slow. The diffusion coefficient is proportional to the square of the distance, and to illustrate how slow the diffusion actually is: an antibody uses more than 10 days to diffuse 1cm.
The interstitium or extracellular matrix consists of a protein network embedded in a hydrophilic gel of glycosaminoglycans and proteoglycans. Collagen is the most important structural network in the extracellular matrix. It is not clear whether the network of fibrillar collagen or the glycosaminoglycan gel plays the most important role in limiting transport of macromolecules through the extracellular matrix (Davies et al 2002). Modulating the extracellular matrix may facilitate the transport of macromolecules thereby increasing the tumour uptake (Brekken et al 2004, davies et al 2004, Eikenes et al 2004, 2005).
A prerequisite for successful therapy is that the therapeutic agents reach the tumour cells. It is therefore of importance to study how the therapeutic molecules penetrate and localise in tumour tissue and micrometastasis. This project is an important supplement to ongoing clinical studies.
The overall aims are:
An important tool in our research is molecular and functional imaging. Centre for Molecular Imaging, Faculty of Natural Science and Technology, NTNU, provide necessary instrumentation.

Intravital microscopy of tumour tissue in transparent window chambers has provided powerful insight into physiological functions such as angiogenesis, metabolic microenvironment, gene expression, and drug delivery in solid tumours (Brown et al 2001). Using confocal laser scanning or multiphoton microscopy, multiple fluorescent molecules can be studied and co-localised simultaneously several hundred micrometers into the sample. Non-invasive measurements require that the tumour grows in transparent window chambers.
Structural information about the tissue, especially the extracellular matrix can be achieved by the second harmonic generated signal (SHG). This is a second order nonlinear optical process, where the emitted light has exactly half the wavelength of the excitation light. No energy is absorbed. The SHG signals depend on the orientation, polarisation and local symmetry properties of the molecules. Collagen is one of the few molecules with properties that generate SHG signals, and the collagen network can thus be visualised without any staining (Brown et al 2003).
Functional parameters such as diffusion of fluorescently labelled molecules are measured by multiphoton excitation and fluorescence recovery after photobleaching (FRAP) (Brown et al 1999), and fluorescence correlation spectroscopy (FCS) (Alexandrakis et al 2004).
- Improve the uptake and distribution of macromolecules by modulation of the extracellular matrix. The extracellular matrix is changed chemically by collagenase and hyaluronidase which degrade the extracellular matrix (Brekken et al 2000b, Eikenes et al 2004, 2005); or by ionising radiation which induces apoptosis (Davies et al 2004).
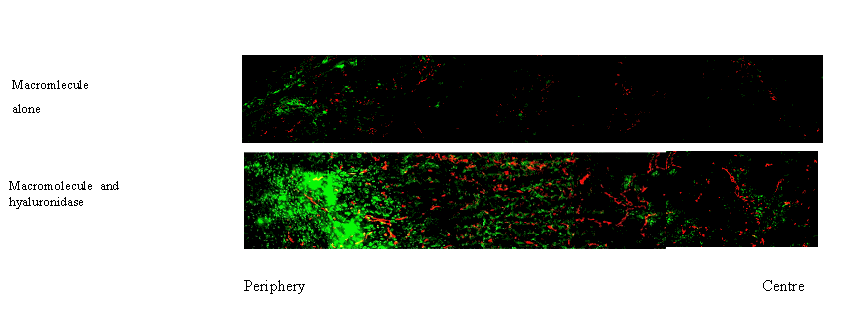
-Study the effect of modulation of extracellular matrix on the two main transport mechanisms: convection (measure interstitial fluid pressure and microvascular pressure) and diffusion (measured by fluorescence recovery after photobleaching or fluorescence correlation spectroscopy).
-Study the impact of the structure of extracellular matrix on uptake and distribution of macromolecules. The collagen network is imaged using the second harmonic generated signal (SHG).
-Study the vasculare network and perfusion, and the effect of modulation of extracellular matrix on the vascularization.
Alexandrakis G, Brown EB, Tong RT, Mckee TD, Campbell RB, Boucher Y, Jain RK.. Nature Med. 10, 203-207, 2004.
Brown E.B., Wu, E.S., Zipfel, W., Webb, W.W. Biophysical J, 77, 2837-2849, 1999.
Brown E.B., Campbell R.B., Tsuzuki Y., Xu L., Carmeliet P., Fukumura D., Jain R.K., Nature Med, 7, 864-868, 2001.
Brown E.B., MvKee, T., diTomaso, E., Pluen, A., Seed, A., Boucher, Y., Jain, R.K. Nature Med. 9, 796-800, 2003.
Boucher Y., Baxter L.T., and R.K, Jain. Cancer Res. 50, 4478-4484, 1990.
Boucher Y., and Jain, R.K., Cancer Res., 52, 5110-5114, 1992.
Brekken, C., Bruland Ø.S., and Davies C. de L. Anticancer Res 20, 3503-3512, 2000a.
Brekken C., Hjelstuen, M.H., Bruland, Ø.S., and Davies C. de L: Anticacer Res. 20, 3513-3520, 2000b.
Davies, C. de L., Berk, D.A., Pluen, A., Jain R.K. Br. J. Cancer, 86, 1639-1644, 2002.
Davies C. de L., Lundstrøm L., Frengen J., Eikenes L., Bruland Ø.S., Kaalhus, O, Hjelstuen MHB, Brekken, C. Cancer Res 64, 547-553, 2004.
Eikenes L., Bruland Ø.S., Brekken C, Davies, C. de L. Cancer Res 64, 4768-4773, 2004.
Eikenes L, Tari, M. Tufto, I., Bruland ØS, Davies C. DeL. Br.J.Cancer 93, 81-88, 2005.
Jain R.K. Cancer Res. 48, 2641-2658, 1988.
Jain R.K. Adv Drug Deliv Rev, 46: 149-168. 2001
Yuan, F. Seminars Radiat. Oncol. 8, 164-175, 1998